57+ calculate the ratio of effusion rates for 235u and 238u.
Web The present-day 238U235U ratio has fundamental implications for uranium-lead geochronology and cosmochronology. Web 125P Calculate the ratio of rates of effusion of 235 UF 6 and 238 UF 6 where 235 U and 238 U are isotopes of uranium.
What Are The Specific Uses Of Uranium 238 The Particular Isotope With Its Benefits And The Careers That Use It Quora
Web Calculate the ratio of rates of effusion of 235UF6 and 238UF6 where 235U and 238U are isotopes of uranium.

. Web The ratio of the rates of diffusion of 235UF 6 and 238UF 6 is. Calculate the ratio of effusion rates for. Web Calculate the ratio of rates of effusion of 235 UF 6 and 238 UF 6 where 235 U and 238 U are isotopes of uranium.
This system is particularly useful because it. The atomic mass of U-235 is 235054 amu and that of U-238 is 238051 amu. Web Calculate the ratio of rates of effusion of 235UF6 and 238UF6 where 235U and 238U are isotopes of uranium.
Web The Two Isotopes Of Uranium 238U And 235U Can Be Separated By Effusion Of Thecorresponding Uf6 Gases. Web Calculate The Ratio Of Effusion Rates For 235U And 238U. The atomic masses are 235U 23504 amu.
Web Calculate the ratio of effusion rates for 238uf6 and 235uf6. The atomic weights are 235 U 23504 amu. Molecular Effusion and Diffusion Last updated Save as PDF Page ID 21766 Learning Objectives To.
Web calculate the ratio of effusion rates for 235u and 238u is available in our book collection an online access to it is set as public so you can get it instantly. Web The two isotopes of uranium 238U and 235U can be separated by effusion of the corresponding U F 6 gases. A 3 B 155 C 1414 D 1004 Hard Solution Verified by Toppr Correct option is D According to Grahams Law for.
The atomic mass of u-235 is 235054 amu and that of u-238 is 238051 amu. Web Calculate the ratio of effusion rates for 238UF6 and 235UF6. The atomic weights are 235 U 23504 amu.
Question Transcribed Image Text. The atomic mass of u-235 is 235054 amu and that of u-238 is 238051 amu. Web Calculate the ratio of effusion rates for 238uf6 and 235uf6.
1086 As discussed in the. What is the ratio in the form of a decimal of the RMS. Aug 28 2022Part a calculate the ratio of effusion rates for 238 uf6 and 235 uf6.
The atomic weights are 235U 23504 amu. Web Calculate the ratio of effusion rates for 235U and 238U and compare it to the ratio for UF6 given in the essay.

Solved Calculate The Ratio Of Rates Of Effusion Of 235 Uf6 And 238 Uf6 Where 235 U And 238 U Are Isotopes Of Uranium The Atomic Masses Are 235 U 235 04 Amu

Calculating The Rate Of Effusion For A Gas Youtube
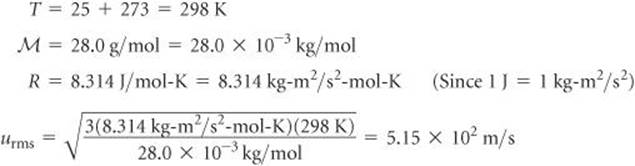
Molecular Effusion And Diffusion Gases Chemistry The Central Science

Solved Calculate The Ratio Of The Effusion Rate Of Oxygen Chegg Com

Uranium Contains Two Isotopes U 235 With An Atomic Mas Of 235 G Mol And U 238 With An Atomic Mass Brainly Com
Solved Molecular Speeds Effusion Obtain The Ratio Of Rates Of Effusion Of H2 And H2se Under The Same

Chapter 5 Converted 1 Pdf Module General Chemistry Chapter 5 Gases Objectives At The End Of The Chapter The Students Will Be Able To 1 Define Physics2 Course Hero
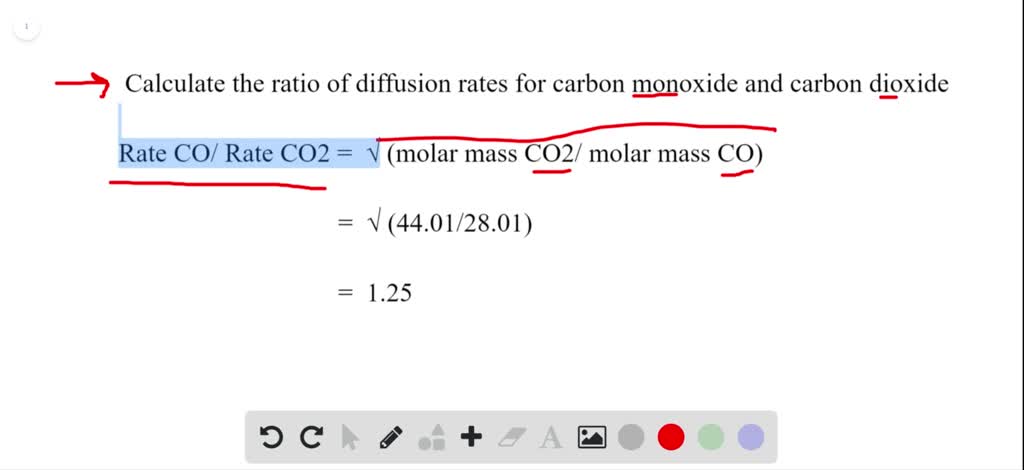
Solved Calculate The Ratio Of Diffusion Rates For Carbon Monoxide And Carbon Dioxide

Periodicity Of Karoo Rift Zone Magmatism Inferred From Zircon Ages Of Silicic Rocks Implications For The Origin And Environmental Impact Of The Large Igneous Province Sciencedirect
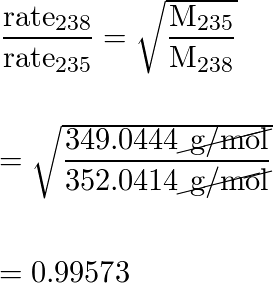
We Separate U 235 From U 238 By Fluorinating A Sample Of Ura Quizlet
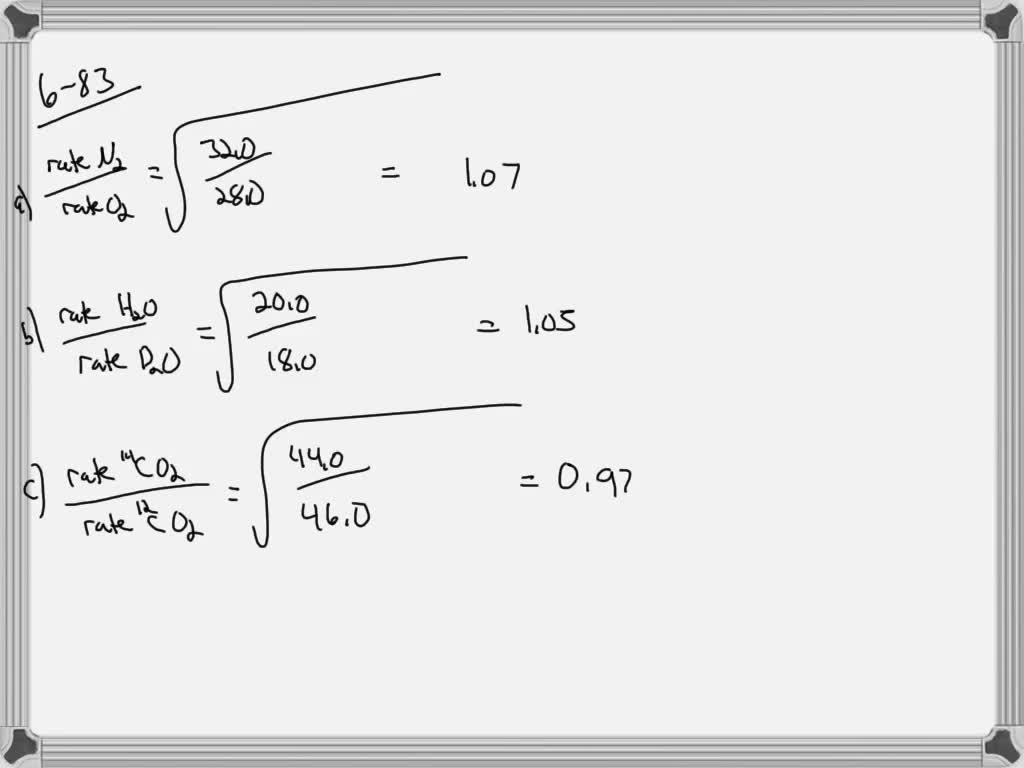
Solved What Are The Ratios Of The Diffusion Rates For The Pairs Of Gases A N2 And O2 B H2 O And D2 O D Deuterium I E 1 2 H C 14 Co2 And
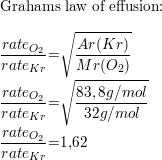
What Is The Ratio Of Effusion Rates For O2 And Kr Quizlet

Bansal Classes Chemistry Study Material For Iit Jee By S Dharmaraj Issuu

Test Diffusion Of Gases 22 Questions Mcq Test Chemistry Class 11 Neet
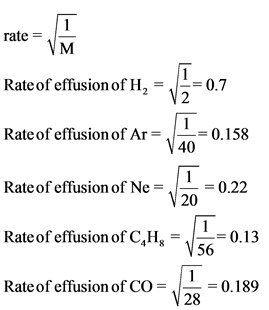
Rank From The Highest To Lowest Effusion Rate To Rank Items As Equivalent Overlap Them Home Work Help Learn Cbse Forum
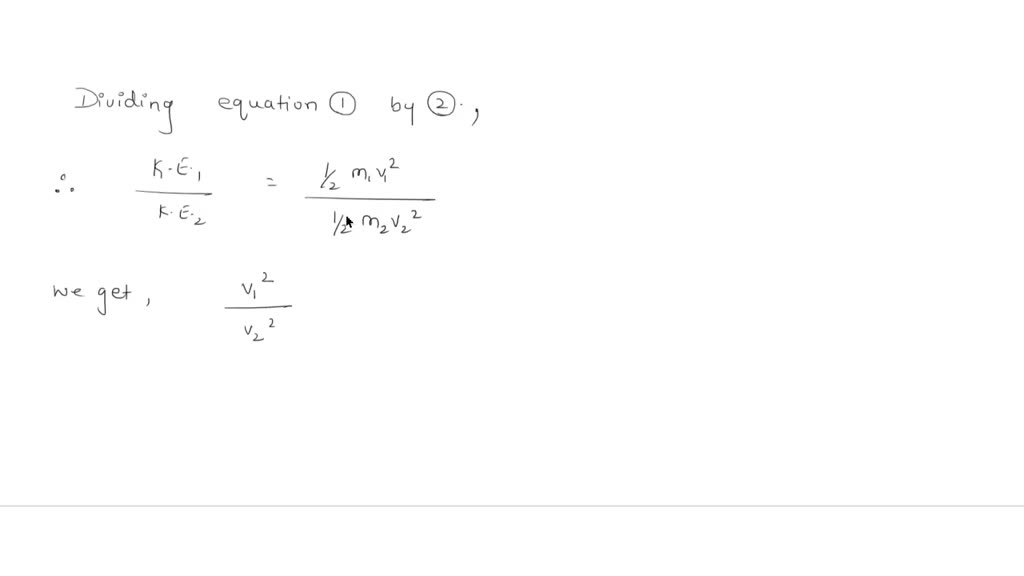
Solved Pan A Calculate The Ratio Of Effusion Rates For 4he And He The Atomic Mass F He 3 Is 3 01603 Amu And That Of He 4 Is 4 00260 Amu Express Your

Calaulate Relative Rates Of Effusion Of O2 To Ch4 Through A Container Containing O2 And Ch4 In 3 2 Mass Ratio